 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free! Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
| Cat. No. | Especies | Descripción del producto | Estructura | Pureza | Característica |
|---|---|---|---|---|---|
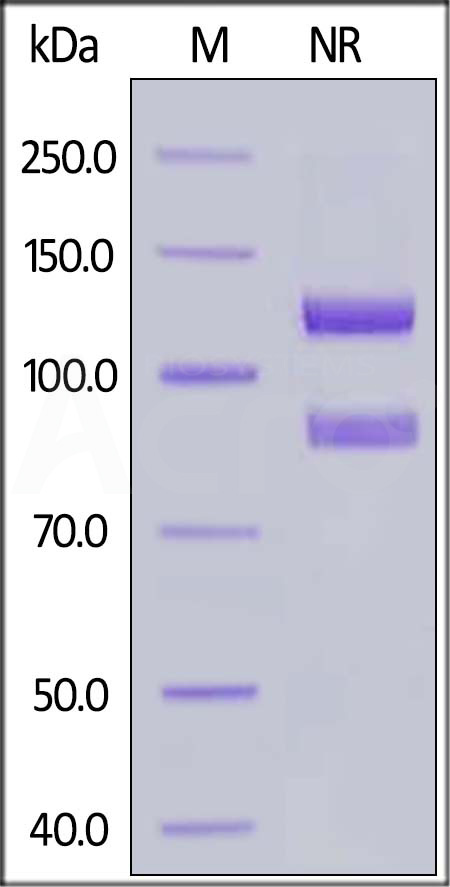
| IT3-H52W8 | Human | Human Integrin alpha 2b beta 3 (ITGAIIb&ITGB3) Heterodimer Protein, His Tag&Tag Free | 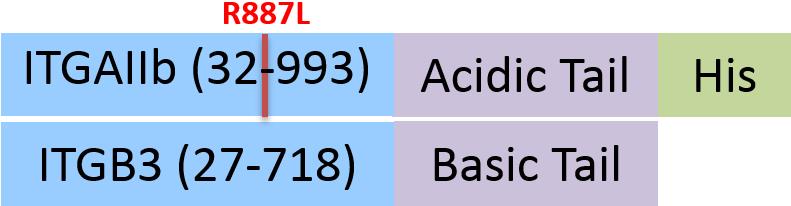 |
|
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Tirofiban Hydrochloride Hydrate | L-462; MK-383; MK-0383; L-700462 | Approved | Merck Sharp & Dohme Corp | 欣维宁, 艾卡特, Aggrastat | United States | Acute Coronary Syndrome; Thrombosis; Coronary Thrombosis | Medicure Inc | 1998-05-14 | Renal Insufficiency; Angina, Unstable; Myocardial Infarction; Stroke; Acute Coronary Syndrome; Vascular Diseases; Coronary Thrombosis; Thrombosis | Details |
| Abciximab biosimilar (ISU Abxis) | ISU-301 | Approved | Isu Abxis Co Ltd | Clotinab, Faximab | Myocardial Ischemia; Myocardial Infarction | Details | ||||
| Abciximab | c7E3-Fab; c7E3-F(ab)2 | Approved | Eli Lilly And Company, Johnson & Johnson Innovative Medicine | ReoPro | United States | Myocardial Ischemia | Janssen Biotech Inc | 1994-12-22 | Myocardial Ischemia; Brain Ischemia; ST Elevation Myocardial Infarction; Myocardial Infarction; Arterial Occlusive Diseases; Angina, Unstable; Stroke; Acute Disease; Carotid Stenosis; Constriction, Pathologic; Renal Artery Obstruction | Details |
| Abciximab biosimilar (Reliance Life Sciences) | R-TPR-019 | Approved | Reliance Life Sciences | AbcixiRel | India | Angina, Unstable; Myocardial Ischemia | Reliance Life Sciences | 2013-01-01 | Myocardial Ischemia; Angina, Unstable | Details |
| Eptifibatide | SB-1; C68-22; Sch-60936; CN-102702321 | Approved | Millennium Pharmaceuticals Inc | Integrelin (former Brand Name), Integrilin | EU | Myocardial Infarction; Angina, Unstable | Glaxosmithkline (Ireland) Ltd | 1998-05-18 | Myocardial Infarction; Angina, Unstable; ST Elevation Myocardial Infarction; Acute Coronary Syndrome; Anemia, Sickle Cell | Details |
| Tirofiban Hydrochloride Hydrate | L-462; MK-383; MK-0383; L-700462 | Approved | Merck Sharp & Dohme Corp | 欣维宁, 艾卡特, Aggrastat | United States | Acute Coronary Syndrome; Thrombosis; Coronary Thrombosis | Medicure Inc | 1998-05-14 | Renal Insufficiency; Angina, Unstable; Myocardial Infarction; Stroke; Acute Coronary Syndrome; Vascular Diseases; Coronary Thrombosis; Thrombosis | Details |
| Abciximab biosimilar (ISU Abxis) | ISU-301 | Approved | Isu Abxis Co Ltd | Clotinab, Faximab | Myocardial Ischemia; Myocardial Infarction | Details | ||||
| Abciximab | c7E3-Fab; c7E3-F(ab)2 | Approved | Eli Lilly And Company, Johnson & Johnson Innovative Medicine | ReoPro | United States | Myocardial Ischemia | Janssen Biotech Inc | 1994-12-22 | Myocardial Ischemia; Brain Ischemia; ST Elevation Myocardial Infarction; Myocardial Infarction; Arterial Occlusive Diseases; Angina, Unstable; Stroke; Acute Disease; Carotid Stenosis; Constriction, Pathologic; Renal Artery Obstruction | Details |
| Abciximab biosimilar (Reliance Life Sciences) | R-TPR-019 | Approved | Reliance Life Sciences | AbcixiRel | India | Angina, Unstable; Myocardial Ischemia | Reliance Life Sciences | 2013-01-01 | Myocardial Ischemia; Angina, Unstable | Details |
| Eptifibatide | SB-1; C68-22; Sch-60936; CN-102702321 | Approved | Millennium Pharmaceuticals Inc | Integrelin (former Brand Name), Integrilin | EU | Myocardial Infarction; Angina, Unstable | Glaxosmithkline (Ireland) Ltd | 1998-05-18 | Myocardial Infarction; Angina, Unstable; ST Elevation Myocardial Infarction; Acute Coronary Syndrome; Anemia, Sickle Cell | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Zalunfiban | RUC-4 | Phase 3 Clinical | The Rockefeller University | ST Elevation Myocardial Infarction | Details |
| batifiban | BAT-2094 | Phase 3 Clinical | Bio-Thera Solutions Ltd | Myocardial Ischemia; Myocardial Infarction; Angina, Unstable; Angina Pectoris; Acute Coronary Syndrome; Thrombosis; Non-ST Elevated Myocardial Infarction | Details |
| MT-1002(Shaanxi Micot Technology) | MT-1002 | Phase 2 Clinical | Shaanxi Micot Technology Co Ltd | Blood Coagulation Disorders; ST Elevation Myocardial Infarction; Angina, Unstable; Acute Coronary Syndrome; Stroke; Thrombocytopenia; Non-ST Elevated Myocardial Infarction; Thrombosis | Details |
| BGC-728 | BGC-728 | Phase 2 Clinical | Btg Plc | Myocardial Infarction; Stroke | Details |
| EGT-022 | EG-22; HU-005; EGT-022 | Phase 2 Clinical | Eyegene Inc | Pressure Ulcer | Details |
| HYC-11395 | HYC-11395 | Phase 1 Clinical | Hefei Cosource Medicine Technology, Nanjing Heqi Pharmaceutical Technology Co Ltd | Coronary Artery Disease; Thromboembolism | Details |
| Zalunfiban | RUC-4 | Phase 3 Clinical | The Rockefeller University | ST Elevation Myocardial Infarction | Details |
| batifiban | BAT-2094 | Phase 3 Clinical | Bio-Thera Solutions Ltd | Myocardial Ischemia; Myocardial Infarction; Angina, Unstable; Angina Pectoris; Acute Coronary Syndrome; Thrombosis; Non-ST Elevated Myocardial Infarction | Details |
| MT-1002(Shaanxi Micot Technology) | MT-1002 | Phase 2 Clinical | Shaanxi Micot Technology Co Ltd | Blood Coagulation Disorders; ST Elevation Myocardial Infarction; Angina, Unstable; Acute Coronary Syndrome; Stroke; Thrombocytopenia; Non-ST Elevated Myocardial Infarction; Thrombosis | Details |
| BGC-728 | BGC-728 | Phase 2 Clinical | Btg Plc | Myocardial Infarction; Stroke | Details |
| EGT-022 | EG-22; HU-005; EGT-022 | Phase 2 Clinical | Eyegene Inc | Pressure Ulcer | Details |
| HYC-11395 | HYC-11395 | Phase 1 Clinical | Hefei Cosource Medicine Technology, Nanjing Heqi Pharmaceutical Technology Co Ltd | Coronary Artery Disease; Thromboembolism | Details |
This web search service is supported by Google Inc.
